

Compare the bond angles of the – structure to the ideal bond angles of an octahedral structure. Compare the bond angles on the structure to the ideal VSEPR model angles in the table in the next section. All molecules are treated in the same way.Įxamine the crystal structure in the database to see whether the molecule’s VSEPR shape is right. For PF6 divide the total number of electrons by two to get the total number of electron pairs.įind the basic VSEPR shape allocated to the molecule using the table in the next section and the total number of electron pairs. When this additional electron is added to the other electrons, the total should be 12 electrons. Because phosphorus has a negative charge, an additional electron is present. There are 6 Fluorine atoms in the – molecule, each of which contributes one electron, therefore adding the 6 electrons to the valence shells totals 11 electrons. Each atom connected to the core atom will contribute electrons to it multiply the number of valence shells by the number of additional electrons. Charge electrons by adding or subtracting them and then multiply the sum of these by two to get the total number of electron pairs and at last predict the shape using this number.īecause phosphorus belongs to group 15, it possesses 5 electrons in its outermost shell. After this, find the Substitute of one electron for each bonding atom. The VSEPR rule says that first the central atom must be identified, then one should Determine the number of valence electrons. Phosphorus is the core atom in this molecule (P). Determine the centre atom on the Lewis diagram. To determine the shape of the molecules, first sketch out the molecule’s Lewis structure. The VSEPR predicted shapes of molecules may be obtained systematically by determining the number of electron pairs in the molecules. Using VSEPR Theory to Predict Molecule Shapes The ammonia molecule, on the other hand, has triple bonding electron pairs, one per hydrogen atom, resulting in a trigonal pyramidal structure. While both water and ammonia molecules have four valence shell electron groups, the water molecule consists of two bonding and two nonbonding electron pairs, resulting in a v-shaped molecule as the two hydrogen atoms are pulled closer together to account for the two nonbonding electron pairs. These pairs are arranged around the valence shell to attain the greatest feasible distance among them, and only the bonding electron pairs, or those linked to an atom, contribute to the final structure of the molecule.Īn illustration related to this, CO2 is a linear molecule because it has two bonding electron pairs and no nonbonding electron pairs. Using a rough draft of a molecule’s Lewis dot structure, which employs dots to denote the amount of valence, or outer shell, electrons that each included atom possesses, you may count the number of bonding and nonbonding electron groups that ring the central atom. It is important to note, however, not all inorganic compounds have the exact shape of molecular geometry. The VSEPR notation provides a generic formula for categorizing inorganic compounds depending on the number of electron pairs around a core atom. Whenever the electron groups are all bond pairs, they are all called in the same way as the electron-group geometry. Electron-group geometry is governed by the number of electron groups, while Molecular geometry is defined by the number of lone pairs as well as the number of electron groups. This is divided into two categories: electron-group geometry and molecular geometry. As a result, the form of the molecule represents its equilibrium state, in which it has the lowest possible energy in the system.Īlthough VSEPR theory predicts electron dispersion, we must take into account the real determinants of molecule shape. The electrons and nuclei gravitate towards the direction of places that reduce the effect of repulsion and promote attraction. The arrangement of a molecule’s nucleus and electrons decides its form. The electron bond pairs and lone pairs on the core atom can help to forecast the structure of a molecule using the VSEPR theory. An electron group on the core atom might be an electron pair, a lone pair, a single unpaired electron, a double bond, or a triple bond. VSEPR theory is concerned not just with electron pairs, but also with electron groups overall.
/AX3E0-side-2D-56a1339e5f9b58b7d0bcfd37.png)
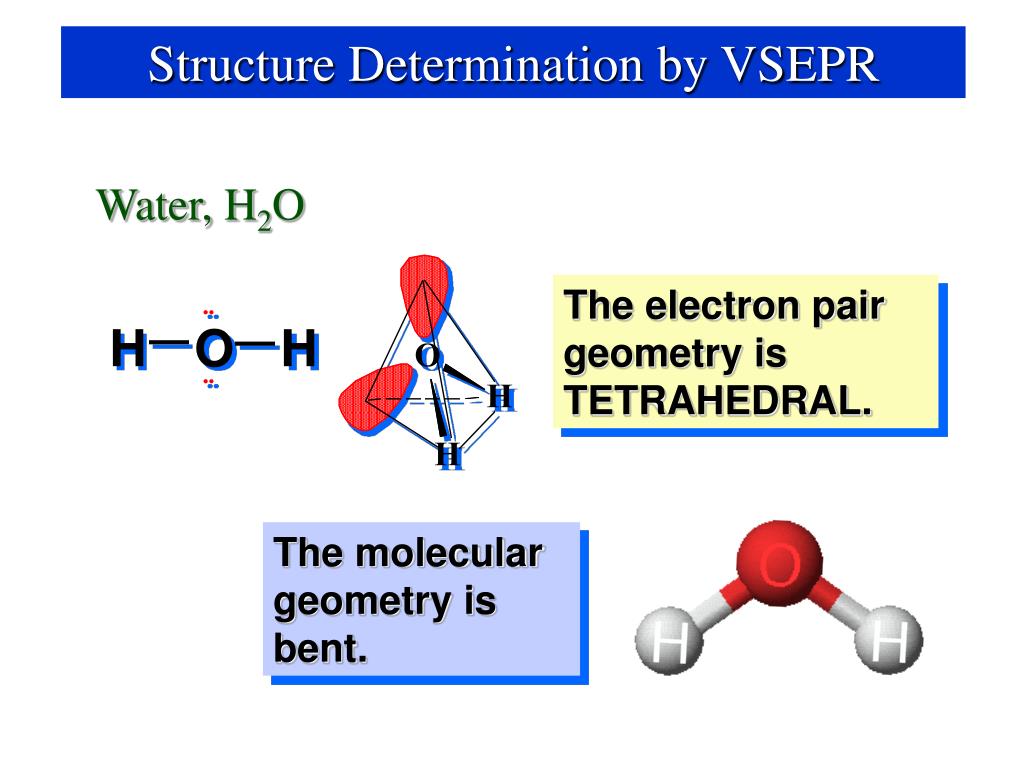
As a result, electron pairs will spread themselves as far apart as possible to decrease repulsion. VSEPR theory definition states that in valence-shell electron-pair repulsion (VSEPR) theory, electron pairs repel one another whether they are in bond pairs or lone pairs.


 0 kommentar(er)
0 kommentar(er)
